What is the lewis structure for NCl3. The other two chlorines have one single bond and three lone pairs of electrons.

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube
Draw the Lewis structure of NCl3.
. Start your trial now. Find the total number of valence electrons in a molecule- Adding up the valence electrons of all the atoms in a molecule is the first step. Include lone pairs and formal charges.
Draw the Lewis structure for NCl3. 3 on a question Draw the Lewis structure of NCl3NCl3. Include lone pairs on all atoms where appropriate.
Up to 256 cash back Get the detailed answer. The bond angles are Cl-N-Cl. Give some similar molecules to PCl 3 lewis structure.
How many lone pairs are there on PCl 3 lewis structure. In the image the red color dots are the valence electrons of. They are similar to electron dot diagrams in that the valence electrons in lone pairs are represented as dots but they also contain lines to represent shared pairs in a chemical bond single double triple etc.
A step-by-step explanation of how to draw the COF2 Lewis Dot Structure Carbonyl fluorideFor the COF2 structure use the periodic table to find the total nu. The lone pairs are the electron dots around the oxygen elements. Chemistry questions and answers.
What is the Lewis dot structure for XeF2. There are one lone pair on nitrogen atom and three lone pairs on each chlorine atom. One chlorine has one double bond and two lone pairs of electrons.
From three oxygen atoms one oxygen atom has one lone pair. Also one oxygen atom has a -1 charge and another oxygen atom has a 1 charge. There are several steps to draw the lewis structure of O 3.
The given molecule is As we know that nitrogen has 5 valence electrons and chlorine has 7 valence electrons. See the answer See the answer done loading. Draw the Lewis structure of the following molecule.
Steps of drawing lewis structure of O 3. Start your trial now. Draw the Lewis structure of CO23.
When you follow these steps properly. But each chlorine atom has three lone pairs. Therefore the total number of valence electrons in butyne 1 5 3 7 26.
Find Alternatively a dot method can be used to draw the lewis structure of BF3. Include all lone pairs of electrons. So in PCl 3 lewis structure has 10 lone pairs.
The formula for the formal charge is also given. Part A ClO Draw the Lewis structure for the molecule. Solution for Draw the Lewis structure of HCN.
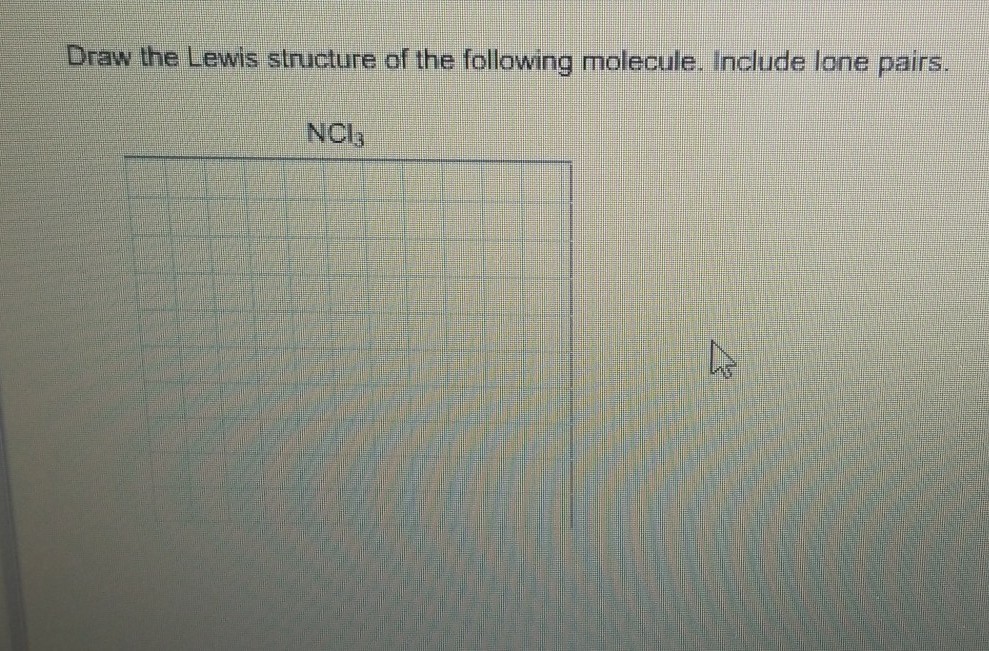
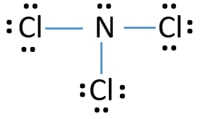
Another one has two lone pairs and remaining one has three lone pairs. Count the valenceSo that is the Lewis dot structure. Nitrogen trichloride NCl 3 lewis structure contains three N-Cl bonds.
Lewis structure of NCl 3 can be drawn by using valence electrons of nitrogen and chlorine atoms. Nitrogen has one single bond two. Using the formula the formal charge for each.
There is only one lone pair on phosphorous atom. The molecule has 26 valence electrons. According to Lewis-dot structure there are 6 number of bonding electrons and 20 number of non-bonding electrons.
Which statement below best describes this Lewis structure. Draw the Lewis structures of the given molecules. OF CO2 Select Draw Rings More Erase Select Draw Rings More Erase.
SubmitMy AnswersGive Up Correct Part B ClO- Draw the Lewis structure for the ion. Now the central atom is generally the least electronegative. First week only 499.
First week only 499. Include lone pairs on all atoms where appropriate. Nitrogen has two single bon.
The Lewis structure for the carbonate ion is attached as shown. Draw the lewis structure of ncl3 include lone pairs Portray the midnight with dark midnight blue along with some smoky grey more than it portraying clouds with these stars peeping out and making use of studs to help make constellations is simply so superb. Now lets see the proper steps to draw a lewis structure-1.
Show the formal charges of all atoms in the correct structure. Draw a skeleton structure. Show transcribed image text.
Around nitrogen there are 4 pairs around it three pairs that bond and one that is a lone pair. Put the least electronegative atom C in the middle with H and Cl on either side. Draw the Lewis structure of COCO.
How to Draw a Lewis Structure for NCl3. Formal charges are optional. The Lewis structure was named after Gilbert N.
Therefore there are total of 9 lone pairs on 3 chlorine atoms. Choose a central atom and draw a skeletal structure- Sketch a skeletal of the molecule with only single bonds. Draw the Lewis structures of the given molecules.
Lewis who introduced it in his 1916 article The Atom and the Molecule. Solution for Draw the Lewis structure of NCl3.

The Total Number Of Lone Pairs In Ncl3 Is Study Com
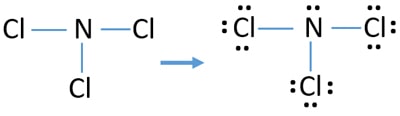
Ncl3 Nitrogen Trichloride Lewis Structure

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

Draw The Lewis Structure Of The Following Molecule Include Lone Pairs Ncl3 Brainly Com

Ncl3 Lewis Structure Molecular Geometry Bond Angle Polarity Electrons
Ncl3 Nitrogen Trichloride Lewis Structure

Solved Draw The Lewis Structure Of Nci Include Lone Pairs Chegg Com

Solved Draw The Lewis Structure Of The Following Molecule Chegg Com
0 comments
Post a Comment